
NASDAQ: INM
Redefining the Future of Alzheimer’s & Neurodegenerative Treatment
Investment Highlights
InMed is a pharmaceutical company focused on developing a pipeline of proprietary small molecule drug candidates targeting the CB1/CB2 receptors
• Multi-program pipeline targeting high unmet-need indications — Alzheimer’s, Dry AMD (ocular), and Dermatology
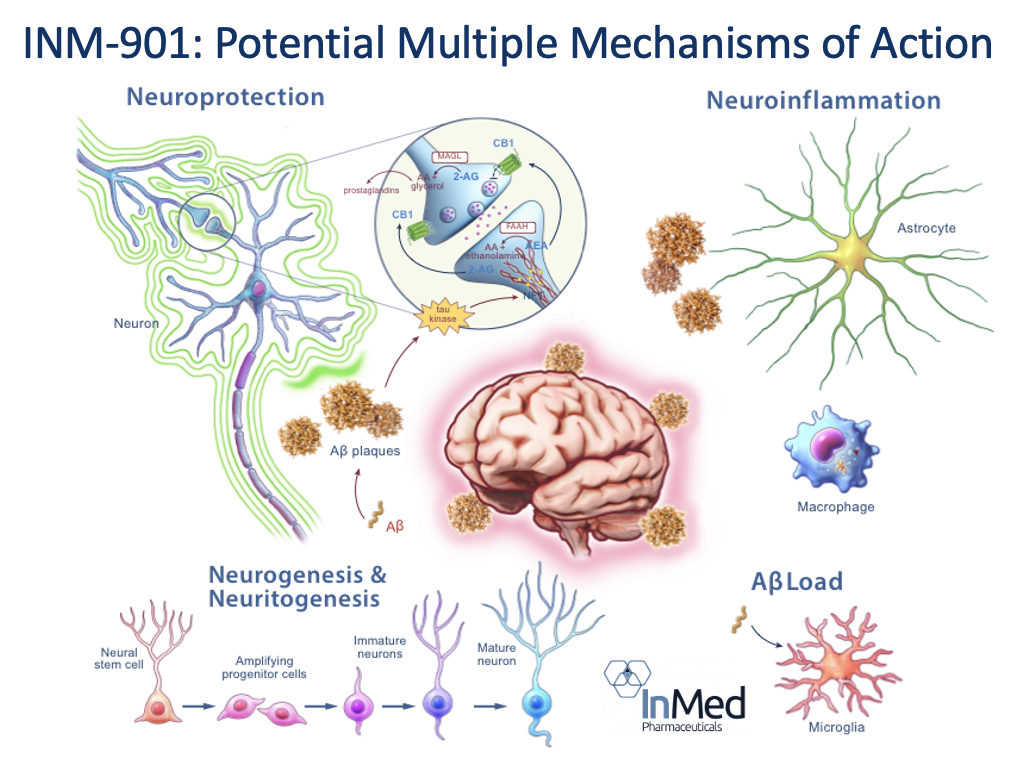
• Lead candidate INM-901: Oral small molecule for Alzheimer’s that crosses the blood-brain barrier, reduces neuroinflammation, and shows cognitive improvements in preclinical studies
• Undervalued opportunity: ~$9M market cap sitting below current cash position, offering full pharma pipeline + revenue stream at virtually no enterprise value
• Strong management team with deep experience in biotech drug development, commercialization, and capital markets
• Cash runway into late 2026 limiting near-term dilution risk
Pipeline Overview
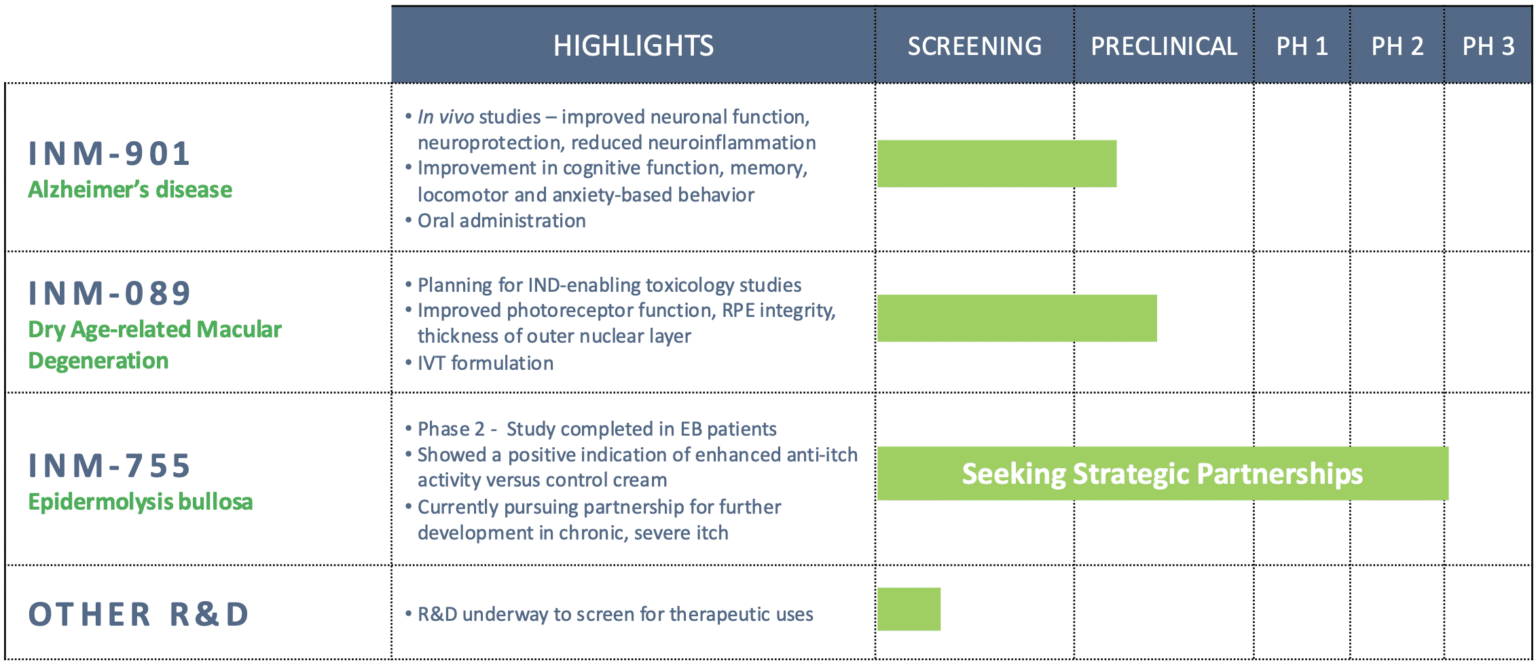
INM-901 — Alzheimer’s Disease
• Oral small molecule that crosses the blood-brain barrier
• Reduces neuroinflammation, promotes neurite outgrowth, enhances neuronal function
• Demonstrated positive trends in cognition, memory, locomotor and anxiety-based behavior
• Aligned on current “beyond amyloid” trend (multi-pathway)
• Targeting a $13B+ and rapidly growing Alzheimer’s market
• Demonstrated statistically significant reduction in several key pro-inflammatory markers
INM-089 — Dry Age-related Macular Degeneration (AMD)
• Proprietary small molecule cannabinoid analog
• Preserves photoreceptor structure and retinal function in preclinical models
• Addresses a leading cause of blindness affecting 150M+ people globally
• No FDA-approved treatments currently exist
INM-755 — Dermatology
• Cannabinol (CBN) cream completed Phase 2 in EB
• Demonstrated clinically meaningful anti-itch activity and safety
• Seeking strategic partners for advancement
